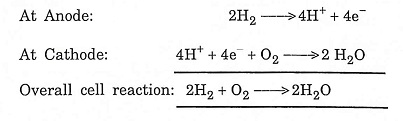Fuel Cell Working Principle and Schematic Diagram:
Fuel Cell Working Principle explains that it is an electrochemical device that converts chemical energy of a conventional fuel directly into low voltage D.C. electrical energy. It is then described as a primary battery in which fuel and oxidizer are stored external to the battery and fed to it when needed.
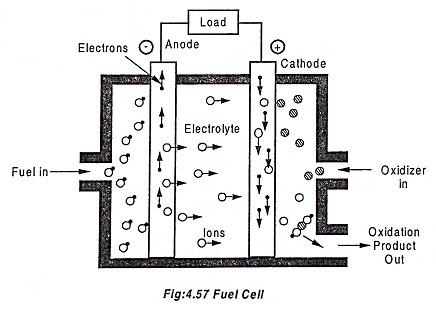
A schematic diagram of fuel cell is shown in Fig.4.57. The fuel gas is diffused through the anode and is oxidized, thus releases electrons to the external circuit. The oxidizer is diffused through the cathode and is reduced by the electrons coming from the anode through the external circuit.
The fuel cell keeps permitting the fuel molecule to mix with the oxidizer molecules, and allow the transfer of electron by a metallic path that contains a load.
Hydrogen-oxygen fuel cell:
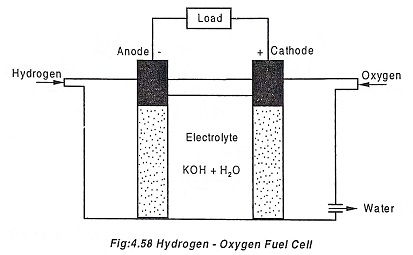
This fuel cell uses hydrogen as fuel and oxygen as an oxidiser. A typical hydrogen-oxygen fuel cell is shown in the Fig.4.58.
There are three chambers separated by two porous electrodes, the anode and cathode. The middle chamber between the two electrodes is filled with electrolyte (strong solution of potassium hydroxide). The electrodes surfaces are chemically treated to repel the electrolyte in order to restrict the flow of potassium hydroxide to the outer chambers.
The gases diffuse through the electrodes by undergoing the following reaction.
When the temperature is high, the electrolyte material acts as a sieve and the hydrogen ions migrates through the material. An electrical load is connected between the anode and the cathode.
The chemical reaction in the cathode, the energy representing the enthalpy of combustion of fuel is released and a part of it is available for conversion into electrical energy.
The water formed is drawn off from the side.
Advantages of fuel cells:
- Conversion efficiency is high.
- Easy and simple construction.
- Require very little attention and maintenance.
- High power to weight ratio.
- Fuel cell does not make any noise.
- Less space required.
- Quick operation.
- Can be installed at the use point.
Disadvantage of fuel cell:
- It is very costly.
- Short service life.
- Low voltage output.
- Proper attention is needed while selection of materials.
Application of fuel cell:
There are numerous applications of fuel cell because of its compact size and easy to handle nature. Some of the main areas of applications are
- Domestic use
- Automotive vehicle
- Central power station