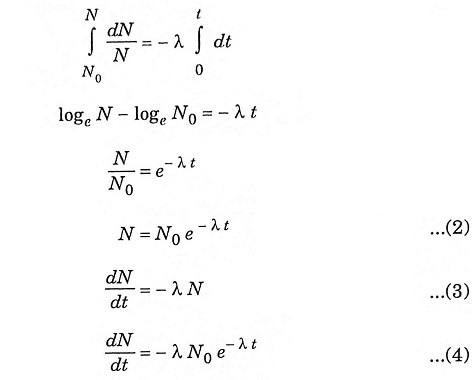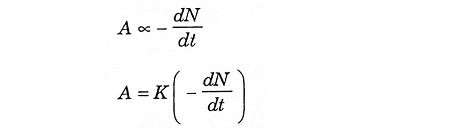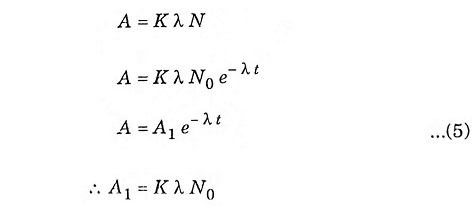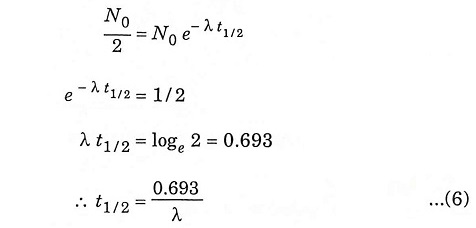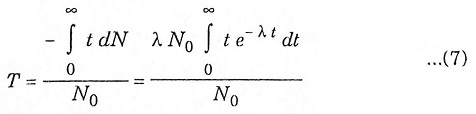What is Radioactivity? – Definition and Types
The phenomenon of spontaneous emission of powerful radiation exhibited by heavy elements is called radioactivity. It is an irreversible self-disintegrating activity as the elements breaks itself up (fission reaction). The elements that exhibit this activity are called radioactive elements. Example of radioactive elements are uranium, radium, thorium, pulutonium, radon, ionium, actinium and mesothorium.
The radioactivity may be natural or artificial. Natural radioactivity is exhibited by elements as found in nature and occurs generally in heavier elements of the periodic table, whereas artificial radioactivity is a modern technique of transmutation of elements that are lighter than those elements that exhibits radioactivity naturally.
The radioactive radiation emitted by the radioactive elements consists of
- α (Alpha) particle
- β (Beta) particle
- γ (Gamma) rays (or) photons
α particles
These are helium nuclei consisting of two protons and two neutrons emitted from both natural and artificial radioactivity. They are highly ionised but cannot penetrate the skin, so are dangerous only if emitted inside the body.
β – particles
These are fast moving electron emitted by radioactive elements. They are more penetrating than α – particles, but can be easily shielded by a few millimeter of wood or aluminium. They can penetrate a little way into human flesh but are generally less dangerous to people than γ – rays. Its exposure results in effects like sunburn, but it is slower to heal.
γ ray
These are high-energy beam much the same as X-rays. They are excited in many radioactive decay and may be very penetrating, therefore requires much substantial shielding. γ – rays are the main hazard to people dealing with sealed radioactive materials used.
Effects of nuclear radiation on matter
When nuclear radiation interacts with any materials, deposits energy in the materials and have various effects. The chemical properties get altered, the crystalline structure may be altered.
Radioactive decay
The emission of the particles in the form of alpha, beta and gamma radiation is not an instantaneous process. For different element the decay time is different, which follows a certain law. The process is independent of the physical and chemical properties of the given isotope at a particular temperature and pressure.
The law states that “small amount of disintegration of the isotope in a small period is directly proportional to the total number or radioactive nuclei and proportionality constant”.
The above law can be stated in form of equation as
where
- N = Total number of nuclei present
- dN = Number of nuclei present at time dt
- dt = Small time interval
- λ = Proportionality constant
Note: – ve sign indicates that N decreases as time increases
Integrating equation (1) with limits, we get
Activity
The intensity of emitted radiation is termed activity. This is directly proportional to the rate of disintegration of the element.
Let A = activity of time t
From equation (3) and (4)
when
- A1 = initial activity
- K= detection coefficient
Half life
Half-life is the time required for half of the parent nuclei to decay.
Putting N = N0/2 and t = t1/2 in equation (2), we get
Average life
Average life denotes the average of total time for which the radioactive nuclei has disintegrated for several half life. It is greater then the half life.
Let T = Average life, then