What is Atomic Bonds and Types?
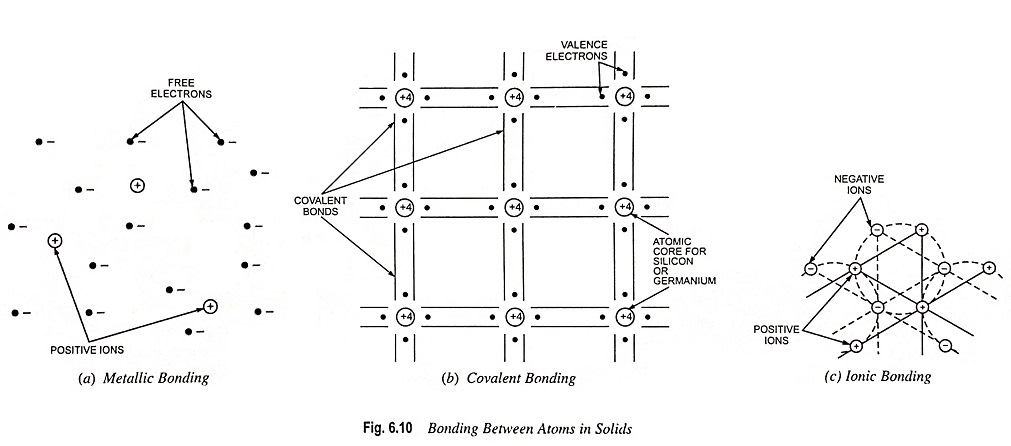
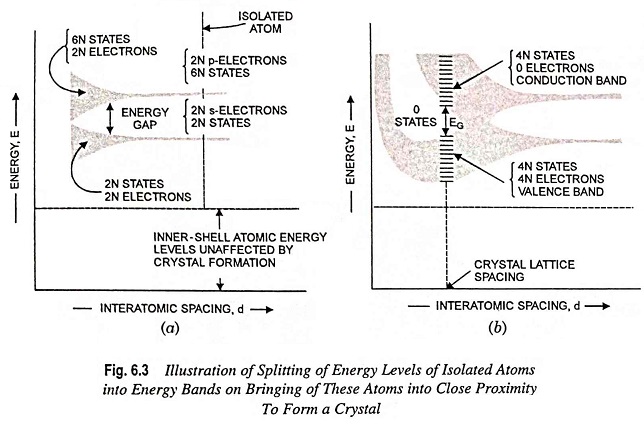
What is Atomic Bonds and Types? Whether a material is a conductor, a semiconductor, or an insulator depends largely upon what happens to the outer-shell electrons when the atoms bond themselves together to form a solid. Atomic bonds arc of two types viz. primary (or chemical) bonds and secondary (or molecular) bonds. Primary bonds are […]
What is Atomic Bonds and Types? Read More »