Extraction of Metals using Electrolysis – Zinc and Aluminium:
Extraction of metals is an electrochemical process used for the production of metal with commercially acceptable purity. There are two methods of extraction of metals depending upon the physical state of the ore. In one of the processes the ore is treated with a strong acid to obtain a salt and the solution of such a salt is electrolysed to liberate the metal. The second process is employed when the ore is available in molten state or can be fused and in this process the ore, which is in a molten/fused state, is electrolysed in a furnace.
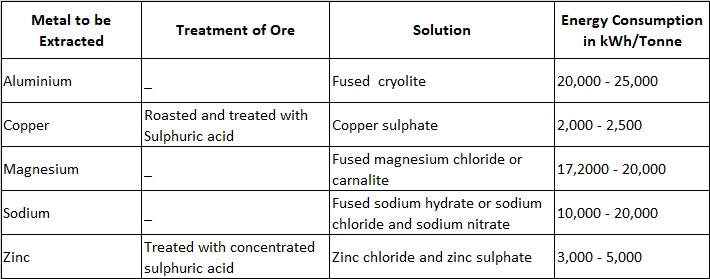
The details of the processes adopted for some of the metals are given in Table 8.2.
The methods adopted for extracting zinc and aluminium are explained below :
1. Extraction of Zinc
This extraction of metals is an example in which an aqueous solution of the salt is used. The ore, consisting largely of zinc oxide, is treated with concentrated sulphuric acid, roasted, and passed through various chemical processes in order to remove impurities (such as cadmium, copper etc.) by precipitation. The zinc sulphate solution so obtained is then electrolysed. The electrolysis of zinc sulphate is accomplished in large lead-lined wooden boxes having a number of aluminium cathodes and lead anodes. Zinc gets deposited on the cathodes and is removed periodically (once or twice a day). The current density on the cathodes is about 1,000 A/m2. The voltage per cell is about 3.5 V and usually 100 to 150 cells are used in series requiring a pressure of roughly 500 V. Energy consumption is 3,000 to 5,000 kWh/tonne.
2. Extraction of Aluminium
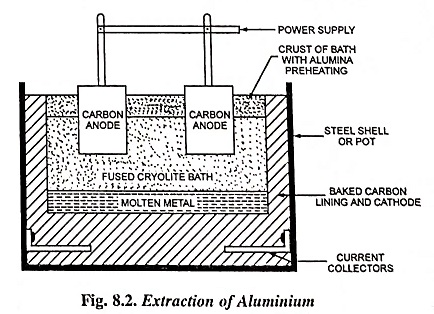
This is an example of fused electrolyte process. Aluminium is produced from bauxite containing aluminium oxide or alumina (70% in case of high grade bauxite), silica (silicon oxide) and iron oxide. The bauxite ore is first reduced to aluminium oxide by chemical treatment and then it is dissolved in fused cryolite. Cryolite is a solution of aluminium fluoride and fluoride of either of sodium, potassium or calcium. The mixture thus obtained is electrolysed. The fusion and electrolysis are accomplished in a large shallow rectangular steel bath lined with carbon; carbon anodes projecting downwards into the bath and the bottom of the bath forms the cathode.
The charge is melted by the arc struck between the carbon anodes and cathode and is then maintained in a molten state by the heating action of the electric current flowing through the charge. The liquid metal deposits at the cathode and settles at the bath bottom and is periodically siphoned out into large ladles from which it is poured into pig or ingot moulds. Fresh alumina is fed into the bath at short intervals to replace that which has been decomposed by the current and the process is, therefore, a continuous one. The aluminium obtained by this extraction of metals process is 99.5% pure.
A furnace having an area of about 15 square metres will need a voltage of about 6 volts and a current of about 40,000 amperes. Energy consumption is 20,000 to 25,000 units/tonne. Almost the whole of the aluminium required in the present day’s industry is produced in this way. As the electrolytic process requires large amount of electric power and process is continuous, so such plants are installed near hydroelectric power stations. The high temperature (1,000°C) necessary to keep the ores in a fused state is maintained by the ohinic losses due to the current flowing through the electrodes and electrolyte.